A tale of ants, jays and nettles
If you have gone for a walk through any European pine forest, you probably have spotted some hills between the trees. Have you looked carefully at those hills? If so, you have seen that these constructions are, in fact, ant hills! In the pine forests of Europe the species Formica rufa is responsible for these ant hills. I enjoy sitting in front of the colonies and observing how the ants work. But be careful! If you get too close they will consider you an enemy and will defend the colony! They will respond by spraying formic acid (Fig. 1)…and jays know it. When a member of this bird species, Garrulus glandarius, spots an ant hill, it disturbs the ants on purpose to get a formic acid bath. Why? Because this compound acts as insecticide (you can observe this curious behaviour here).

Figure 1. Formica rufa defending the nest by spraying formic acid2
As you have suspected, the name formic acid comes from the scientific name of the ant, Formica. This is because this chemical compound was isolated for the first time from ants. John Wray, an English naturalist in 1671 wrote in a letter “If with a staff or other instrument you stir a heap of Ants, so as to anger them, they will let fall thereon a liquor”3. So, in search of more information about that liquor, he distilled some ants until he got what he called an acid spirit.
However, Formica ants are not the only living creatures that produce formic acid. Now maybe you are trying to figure out what other insects do. Here you have another example: Stingless bees (genus Oxytrigona)4. But what do you think if I tell you that plants also synthesise it? There is a common (and extremely hated) plant across Europe that does it: nettle!
I have spent most of my life thinking that when someone is stung by a nettle (Urtica dioica) the stinging hairs, called trichomes, were responsible for the pain (I mean, in a physical way). But the fact is that there is a complex chemical response (a wonderful one) behind that unpleasant experience (Fig. 2). When someone brushes against a nettle, the trichomes pierce the skin so the venom is injected. Formic acid is responsible for inducing the pain5. But wait, nettles have a secret strategy: the three neurotransmitters, histamine, acetylcholine and serotonin, cause inflammation and more pain6,7. Finally, to end this wonderful experience, tartaric and oxalic acid are in charge of extending pain duration8.
So, next time you brush against a nettle, think about it. It won’t be less painful, but at least you will be distracted by their wonder 😉
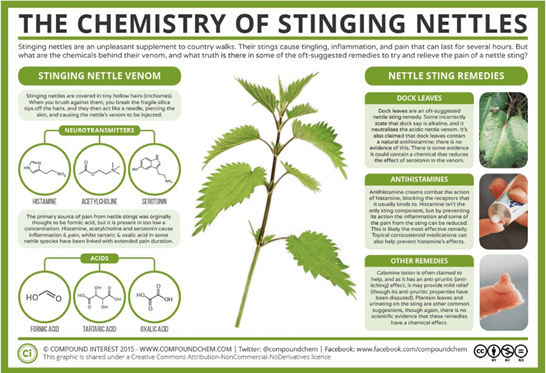
Figure 2. The chemistry of stinging nettles5
Additional information and references
1 https://www.britannica.com/video/180428/nest-ants-formic-acid-jay
2 Warren Photographic. Image Library of Animals in Action
3 Wray, J. (1670). “Extract of a Letter, Written by Mr John Wray to the Publisher January 13. 1670. Concerning Some Un-Common Observations and Experiments Made with an Acid Juyce to be Found in Ants”. Philosophical Transactions of the Royal Society of London, 5(57–68): 2063-2066. doi:10.1098/rstl.1670.0052
4 Roubik, D.W., Smith, B.H. & Carlson, R.G. (1987). Formic acid in caustic cephalic secretions of stingless bee,Oxytrigona (Hymenoptera: Apidae). J Chem Ecol, 13: 1079–1086. https://doi.org/10.1007/BF01020539
5 Dobbin, L. (1920). XI.—On the Presence of Formic Acid in the Stinging Hairs of the Nettle. Proceedings of the Royal Society of Edinburgh, 39: 137-142.
6 Emmelin, N. & Feldberg, W. (1947). The mechanism of the sting of the common nettle (Urtica urens). Journal of Physiology, 106: 440–455.
6 Taskila, K., Saarinan, J.V., Harvima, I.T. & Harvima, R.J. (2000). Histamine and LTC4 in stinging nettle-induced urticaria. Allergy, 55: 680–681.
8 Fu, H.Y., Chen, S.J., Chen, R.F., Ding, W.H., Kuo-Huang, L.L. & Huang, R.N. (2006). Identification of Oxalic Acid and Tartaric Acid as Major Persistent Pain-inducing Toxins in the Stinging Hairs of the Nettle, Urtica thunbergiana, Annals of Botany, 98(1): 57–65. https://doi.org/10.1093/aob/mcl089
9 Compound Interest 2015. https://www.compoundchem.com/2015/06/04/nettles/
🛸 🌎 ° 🌓 • .°• 🚀 ✯
★ * ° 🛰 °· 🪐
. • ° ★ • ☄
▁▂▃▄▅▆▇▇▆▅▄▃▁▂.